June 18, 2024: Clinical Trials, Today and Tomorrow
In January, NIH Director Monica Bertagnolli, M.D., joined our advisory council meeting for a robust discussion of her priorities, including new approaches to clinical research to improve people’s health. We talked about barriers to clinical research, such as lack of trust and trustworthiness, access, and education, as well as the need to get the results to health care settings where they can be used.
Dr. Bertagnolli noted that NCATS is on the forefront of solving challenges like these, and indeed we are.
Our two clinical research networks — the Rare Diseases Clinical Research Network (RDCRN) and the Clinical and Translational Science Awards (CTSA) Program — have been leaders in engaging patients and communities through the entire research process. They focus on different aspects of clinical trial site readiness. The RDCRN strives to de-risk clinical trials for rare diseases. It conducts disease research and natural history studies and works with patient advocacy groups. The CTSA Program offers clinical research expertise, resources, and training. Its partnerships enable a local, regional, and national response to public health needs.
A recent paper summarizes RDCRN’s 20 years of contributions in this space. Here, I’ll focus on three aspects of clinical trial site readiness that the CTSA Program addresses. Its solutions showcase translational science principles in action, particularly finding generalizable solutions and promoting diversity, equity, inclusion, and accessibility (DEIA).
Managing Multisite Trials
Multisite trials can recruit faster and offer larger and more diverse samples, which help ensure that results apply to all who could benefit from a new medicine. However, planning, launching, and running these trials is complex and can take a long time. CTSA Program leaders and other experts published a framework for assessing clinical trial site readiness. This paper and a companion piece emphasizing DEIA can help clinical trial sites be at the top of their game.
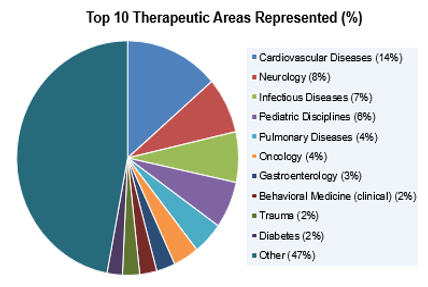
TIN has received 445 requests for support, leading to 37 funded trials. This pie chart shows the top therapeutic areas represented by the initial requests. (NCATS)
Through the CTSA Program, NCATS supports the Trial Innovation Network (TIN). A collaborative national network, TIN has taken great strides toward making clinical trials run better, faster, and more cost-effectively. Its Protocol Assessment Team works with academic and NIH clinical research teams on many facets of trial planning. In recent years, these consults led to 37 funded trials funded by NIH, other federal agencies, industry, and disease foundations. Because of its infrastructure, TIN could quickly power up studies in response to Zika, Ebola, the opioid epidemic, and, most recently, the COVID pandemic. An expanded area of emphasis is enhancing recruitment of diverse individuals in multisite clinical trials. To do this, TIN is engaging with historically Black colleges and universities, minority-serving institutions, and other groups to drive best practices and guidance.
TIN also has been a leader in establishing and advising on electronic health record (EHR)–driven recruitment strategies. The goal of this effort is to enhance research planning, clinical trial design, and enrollment and retention in trials. We expect more progress on this front as the CTSA Program builds upon its strong foundation of real-world data and EHR research — for example, extending efforts to develop clinical decision support tools that the Evolve to Next-Gen Accrual to Clinical Trials Network is helping to drive.
Another roadblock to multicenter clinical trials is establishing a single institutional review board (IRB) that provides ethical review for all the sites. The CTSA Program’s SMART IRB platform provides a broadly shared and agreed-upon IRB reliance agreement that can shorten the IRB step from months to weeks (or, in a few impressive examples, days). More than 1,200 institutions and organizations, including the NIH Intramural Research Program, currently participate in SMART IRB.
Encouraging Active Study Participation
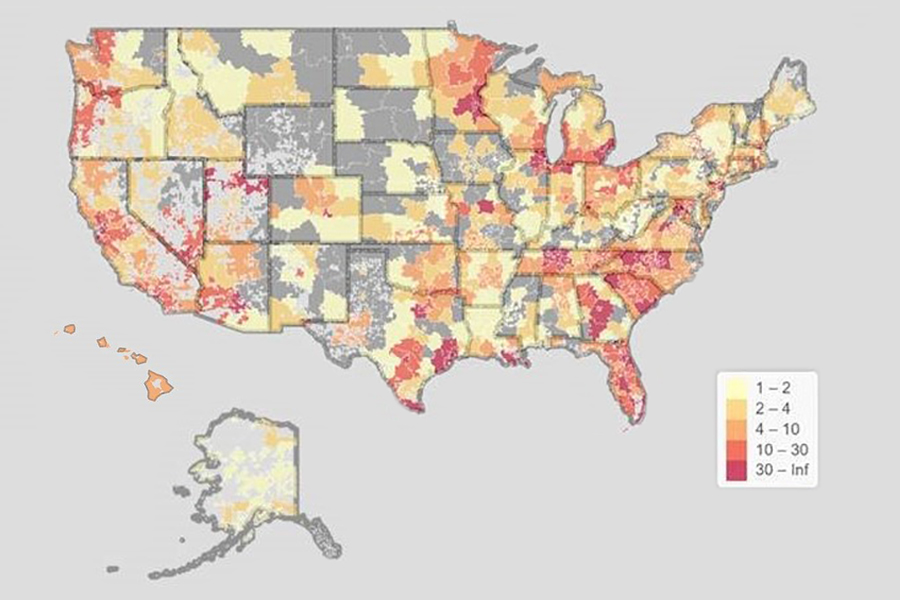
This U.S. map shows the geographic diversity of ACTIV-6 participants across the country. (Dylan Thibault and Chris Lindsell, Duke Clinical Research Institute)
An approach that centers on the participants from the start can make all the difference for a clinical trial. The ACTIV-6 trial that was led by the Duke-Vanderbilt Trial Innovation Center is a great example.
Its decentralized clinical trial (DCT) design sent study medications to participants’ homes. This feature allowed the study to take place entirely remotely and played a great role in ACTIV-6 enrolling more than 10,000 people — making it the highest enrolling trial in the ACTIV program. Plus, participants represented all 50 states, and enrollment of Black or Hispanic participants increased steadily over time.
A key component of ACTIV-6 was a stakeholder advisory committee representing COVID-19 patients, caregivers, and health care providers. The committee, which has been managed by the Patient-Centered Outcomes Research Institute, has met monthly to ensure that partners’ perspectives are included in the research process. It is a model for virtual engagement in DCTs.
DCTs offer a huge opportunity to foster active study participation. Last year, the U.S. Food and Drug Administration took further steps to support the use of DCTs for drugs, biologics, and devices. The report from our 2023 Request for Information on DCTs highlights ways to strengthen and expand the use of DCTs.
Developing the Research Team
A key factor in clinical trial site readiness is having sufficient and diverse personnel. They ensure that the clinical trial enrolls participants who accurately reflect the population affected by the disease or condition being studied. Diversity in the research team also can improve trust.
Every CTSA Program institution contributes to the professional development of a diverse clinical research workforce. The current CTSA Program notice of funding opportunity renews emphasis on this requirement.
To address the training needs of a diverse clinical research workforce, several CSTA Program institutions worked together to create the Development, Implementation and Assessment of Novel Training in Domain-based Competencies (DIAMOND) portal. This crowdsourced catalog of more than 200 trainings covers everything from clinical research design to data management and informatics. Within a few years of its launching, DIAMOND had received nearly 6,000 visits.
Clinical Trials of Tomorrow, Today
All these clinical trial readiness approaches improve the quality, efficiency, and impact of today’s clinical trials. But they also prepare us for the clinical trials of tomorrow, which will have their own challenges that must be carefully managed to ensure the best possible outcomes.
Through the CTSA Program and other initiatives, NCATS is considering needs for supporting new types of precision medicine trials. These trials would include first-in-human studies for new gene-targeted approaches, such as “N of small” gene therapy, gene editing, and antisense oligonucleotide. They also would include precision modeling approaches, such as Clinical Trials on a Chip and in silico or “emulation” trials that use real-world data. In addition, the CTSA Program is expected to serve as a pathway for integrating primary care settings into the broader landscape of clinical research, another priority area for Dr. Bertagnolli.
No matter what the clinical research future holds, keeping “all people” front and center will lead us in the right direction.
Your partner in science and health,
Joni L. Rutter, Ph.D.
Director
National Center for Advancing Translational Sciences


